Sexual selection on condition-dependent traits
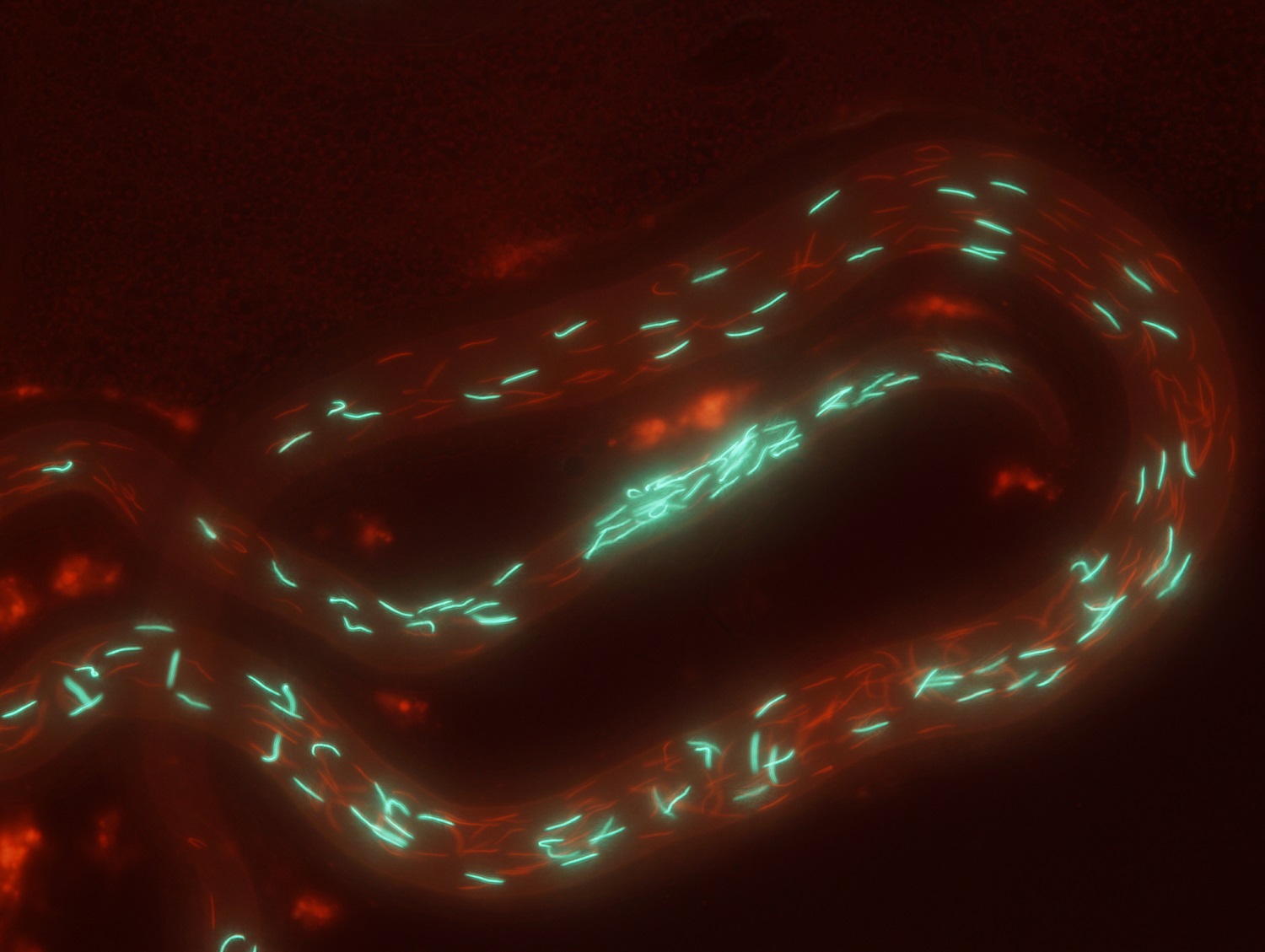
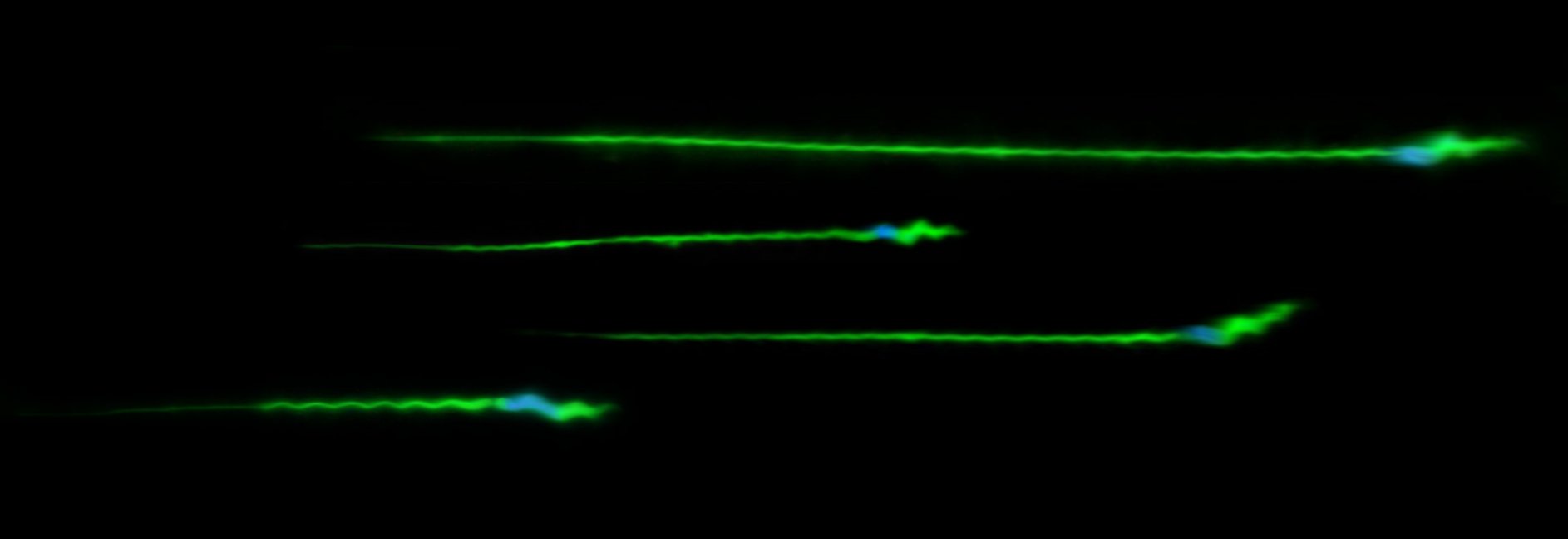
Due to immediate fitness benefits, males tend to maximize their investment in ornaments or armaments involved in mate acquisition, and in ejaculate size and quality that can influence competitive fertilization success among rival males. However, these traits tend to be energetically costly, so that males with limited resources available are constrained in their investment, thereby having a fitness disadvantage. Several theoretical models predict that the condition-dependent expression of such costly traits allows females to assess the underlying genetic quality of males and gain genetic benefits for their offspring when mating with the highest-quality males (those with the most exaggerated traits). However, a genetic link between trait expression and male genetic quality has rarely been tested, and nearly nothing is known about the level of condition dependence in postmating sexual traits and how it relates to fitness outcomes. Using quantitative genetic crossing designs in Drosophila melanogaster as a model system, we are addressing the following goals:
- Quantify genetic variation in male and female quality
- Quantify condition dependence of sexual and non-sexual traits
- Examine intensity of sexual selection on traits relative to their condition dependence and quantify the indirect fitness consequences
In parallel, using cross-generational experiments, we are testing new theoretical models of sexual selection and condition dependence that are based on non-genetic transmission of male condition effects to the next generation.